Since its inception, FIPAN has served as a rallying point for feed millers, animal nutritionists, raw material suppliers, veterinarians, academics, and regulators. The association’s core mission is to professionalize the feed industry, promote high standards, and champion sustainable practices that support livestock development and national food security.
Recognizing the importance of global alignment, FIPAN became a proud member of the International Feed Industry Federation (IFIF), connecting Nigeria’s feed practitioners with global best practices, research, and policy dialogue. This international affiliation has enriched FIPAN’s voice in global fora and strengthened its resolve to elevate Nigeria’s feed industry to international standards.
Over the years, FIPAN has played a strategic role in addressing industry-wide issues such as access to quality raw materials, rising production costs, regulatory clarity, and capacity development. Through its close engagement with development partners and financial institutions, FIPAN continues to facilitate access to resources and opportunities for its members.
From hosting high-level stakeholder summits to training programs and policy advocacy, FIPAN is committed to building a resilient, innovative, and inclusive feed industry. With a growing membership and strong leadership, the association stands as a beacon of progress — ensuring that Nigeria’s feed sector remains a solid foundation for a thriving livestock economy.
The Feed Industry Practitioners Association of Nigeria (FIPAN) is the official trade association of the formal animal feed manufacturing industry in Nigeria. Being a member of the International Feed Industry Federation (IFIF), FIPAN unites prominent industry associations which membership categories are, Feed Manufacturers, Feed Raw Materials Merchants, Feed Toll millers, Feed’s Equipment Manufacturers and Importers, Feed’s Minerals and Vitamins Premix manufacturers, Feed Concentrates and Additives Importers, Feed Raw Materials Vendors, Finish Feeds Distributors and Industry Feed Consultants.
Global Macro-economic Environment
The global economy in 2024–2025 faced persistent challenges, including supply chain disruptions and geopolitical tensions. The International Monetary Fund (IMF) estimated global GDP growth at 3.2% for 2024, with a projected slowdown to 2.9% in 2025, reflecting tighter monetary policies and reduced demand in advanced economies.
Real GDP Growth (%)

Source: International Monetary Fund (Adapted for NAFFP-National Animal Feed and Fodder Policy- Context)
Inflation remained a critical issue, with consumer price inflation in emerging markets and developing economies reaching 9.5% in 2023 and moderating to 7.3% in 2024. In Nigeria, high food and energy prices, driven by global commodity market volatility and domestic supply constraints, increased the inflation rate to 29.9% in 2023 and 24.1% in 2024, with feed costs accounting for 70–75% of livestock production expenses.
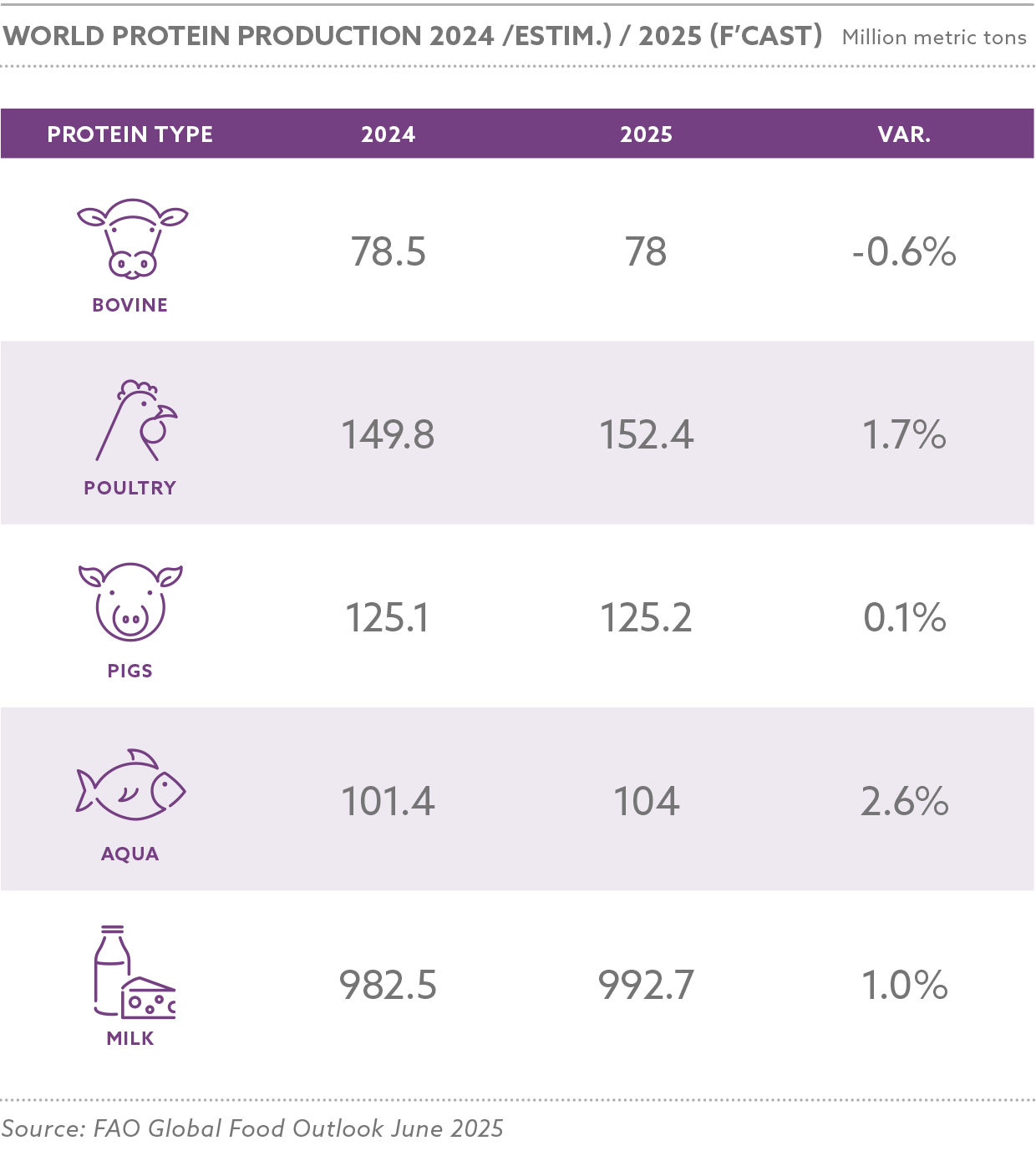
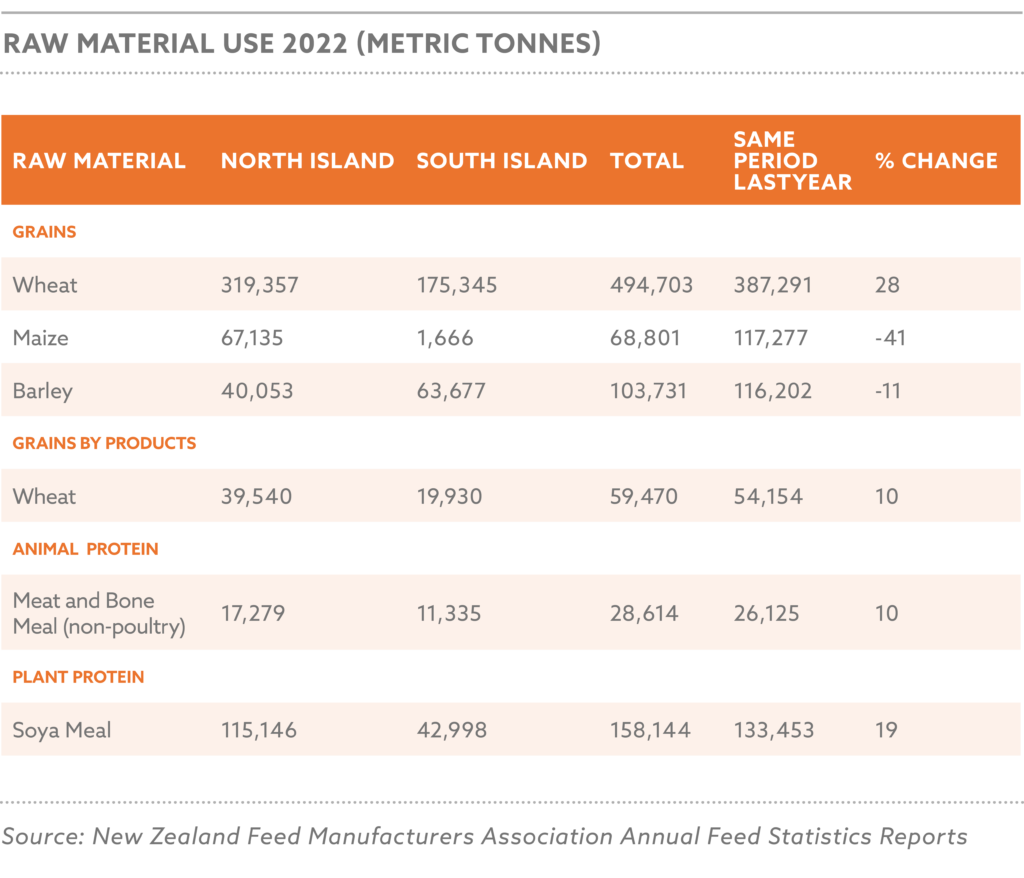
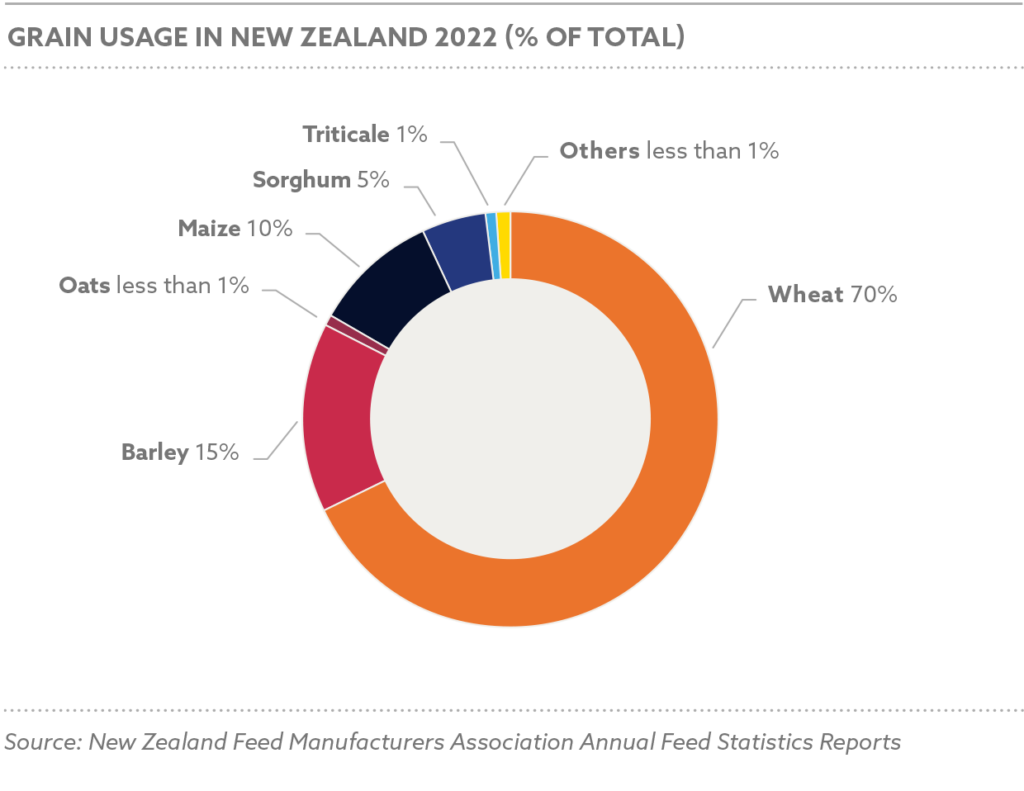
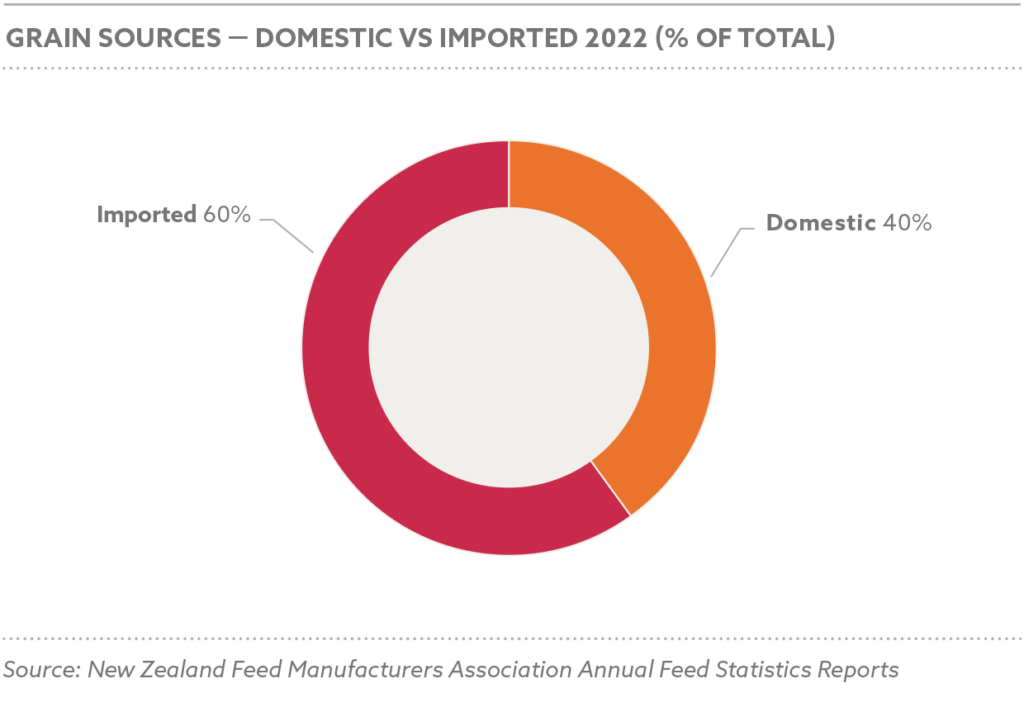
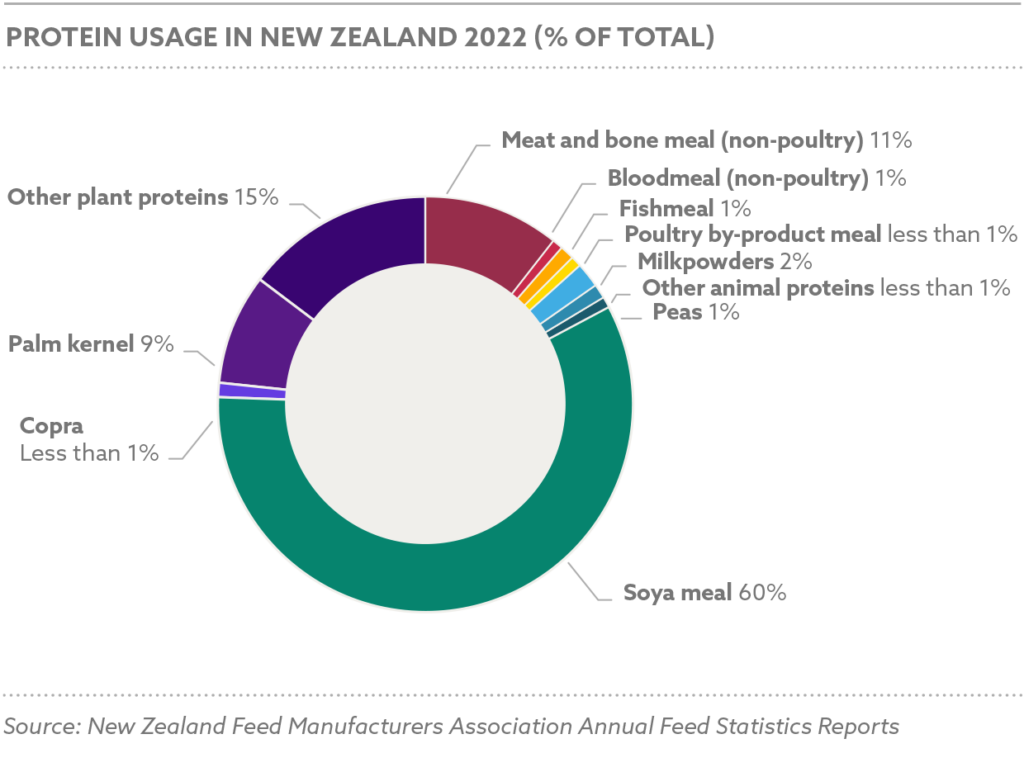
Global grain and oilseed markets faced challenges, with the International Grains Council (IGC) reporting a 2.9% decline in global grain production for 2024/25 due to adverse weather and geopolitical disruptions. Maize production was estimated at 1.2 billion tons, with Nigeria contributing 12.5 million tons, while soybean production reached 369 million tons globally, with Nigeria’s harvest at 0.7 million tons.
Local Macro-economic Conditions
Nigeria’s economy, heavily reliant on agriculture, faced structural challenges, including inadequate infrastructure, electricity outages, and inefficiencies in rail and road networks. The NAFFP (National Animal Feed and Fodder Policy) projects Nigeria’s GDP growth at 3.0% in 2024, slowing to 2.8% in 2025, constrained by these factors and global economic headwinds. The livestock sector, contributing 1.7% to national GDP and 9% to the agricultural value chain, remains a critical economic driver.
Unemployment in Nigeria remained high, with the agricultural sector employing over 35% of the workforce, including 50% of feed industry jobs linked to small and medium enterprises (SMEs). The NAFFP (National Animal Feed and Fodder Policy) aims to create jobs through feed industry expansion, targeting training for 1,000 stakeholders in feed production and nutrition.
The Nigerian Feed Situation
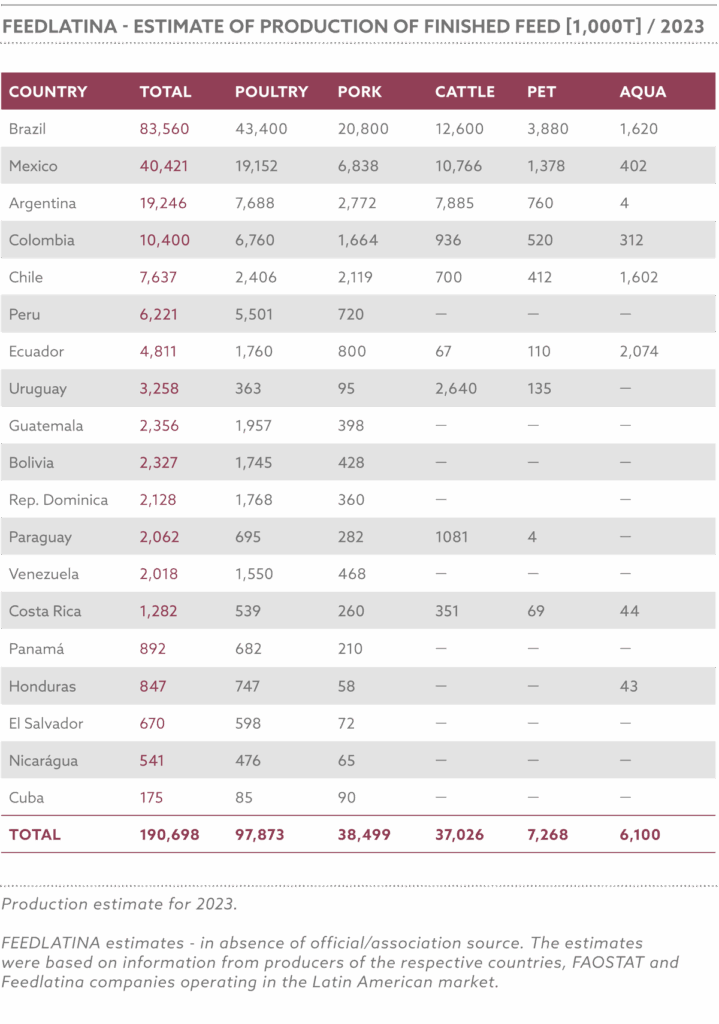
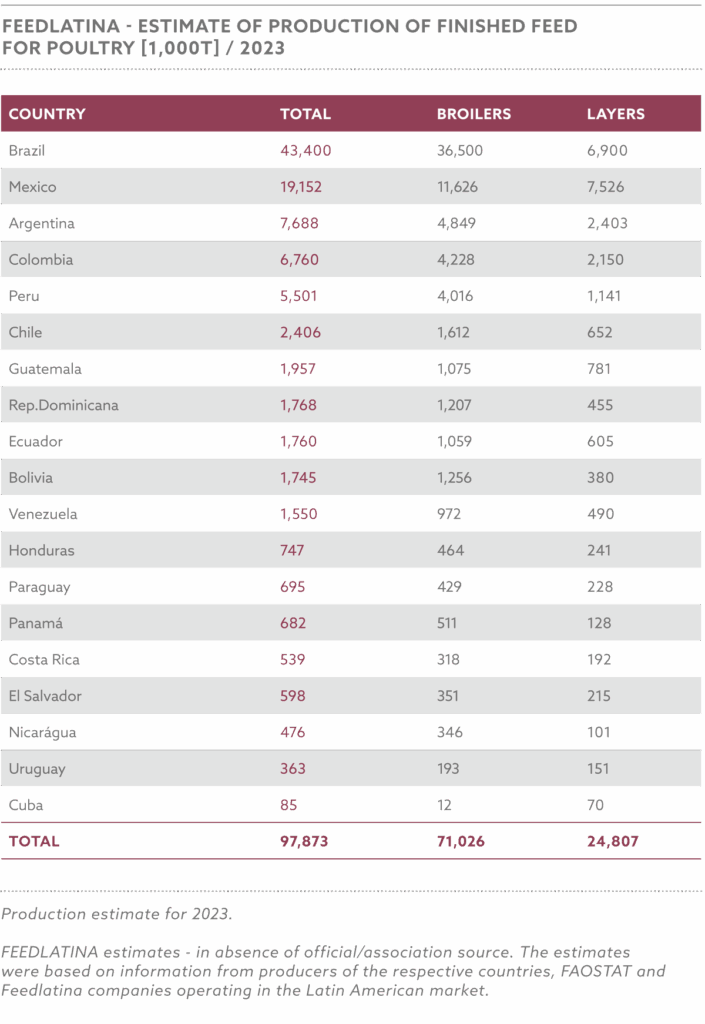
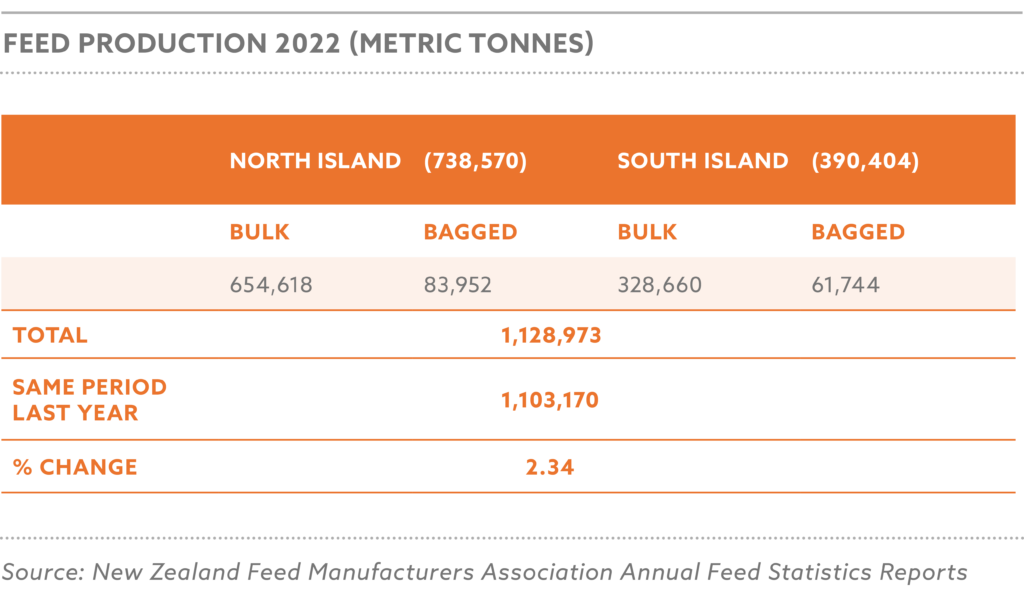
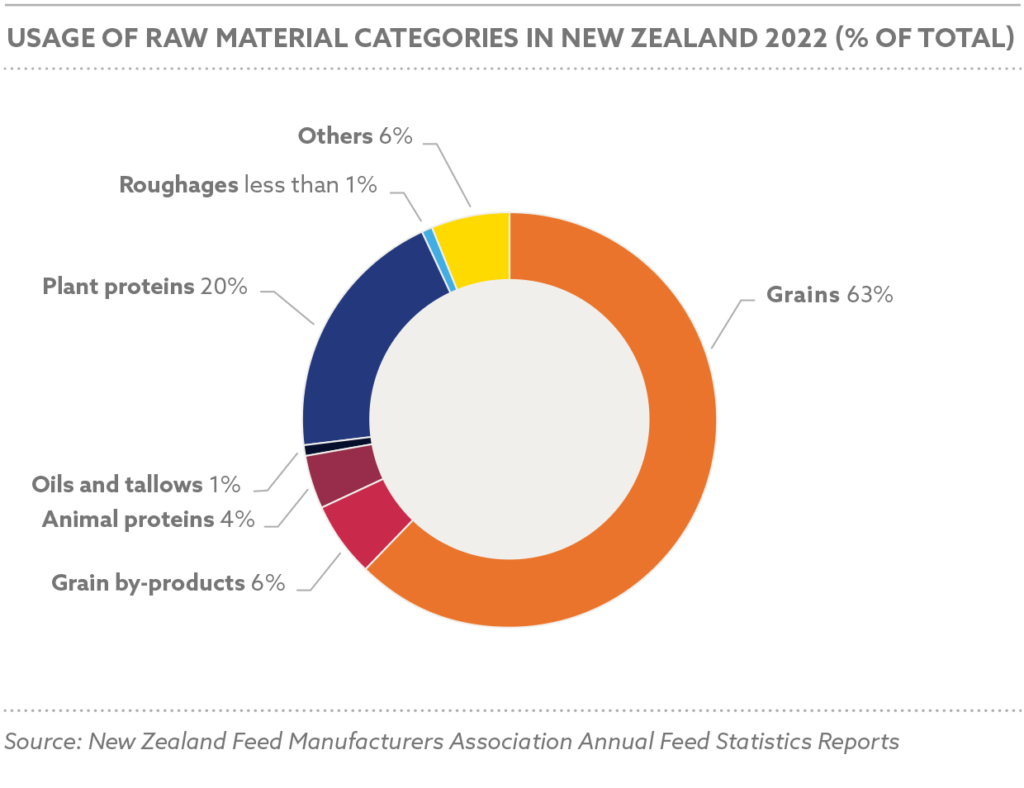
In 2023, Nigeria produced approximately 6.8 million metric tons of animal feed, with an estimated increase to 7.1 million metric tons in 2024, driven by increasing population, urbanization, and government incentives for local feed production, while utilizing 34–36% of its potential 20 million metric ton annual capacity, limited by high production costs, insecurity in agricultural regions, inadequate infrastructure for feed processing, and the devaluation of the naira, which has increased the cost of imported feed inputs
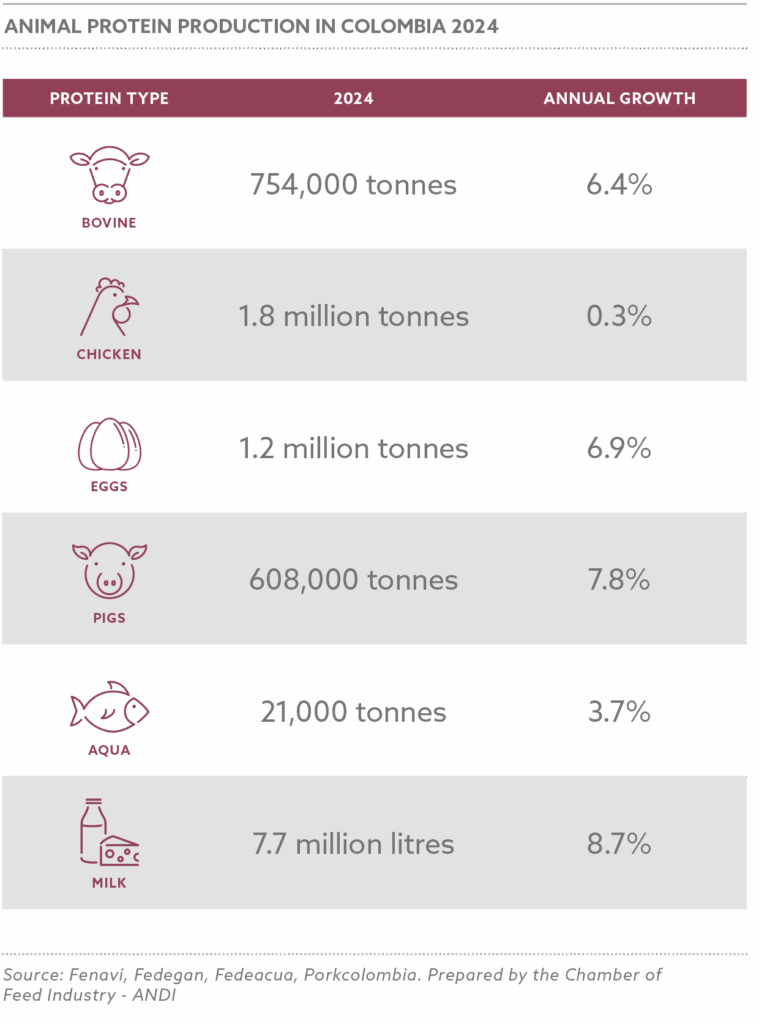
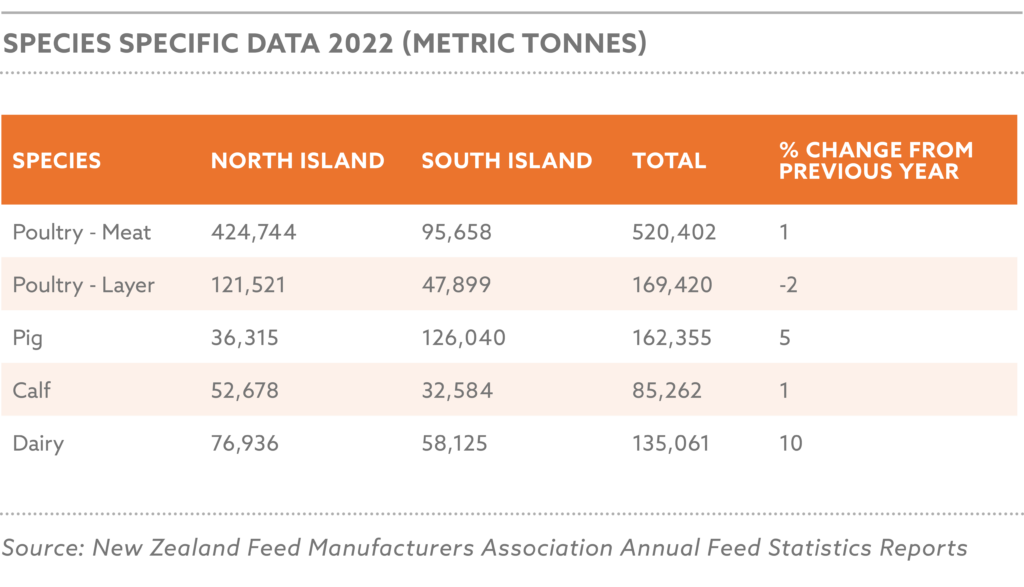
The NAFFP (National Animal Feed and Fodder Policy) targets a production increase to 50 million metric tons by 2030, driven by demand from 16.6 million pigs, 58.7 million cattle, 60.2 million sheep, 108.7 million goats, and 695.5 million poultry, producing 0.5 billion litres of milk, 1.4 million tonnes of meat, and 0.6 million tonnes of eggs annually.

FIPAN-affiliated mills, primarily small-scale operators (82.5% producing ≤2 metric tons/hour), control 60% of the market share, with 650 of 787 surveyed mills in 2014 being small-scale. The policy supports upgrading these mills to medium and large-scale operations, targeting an additional 7 million metric tons for cattle and 10 million for sheep and goats under ranching systems.
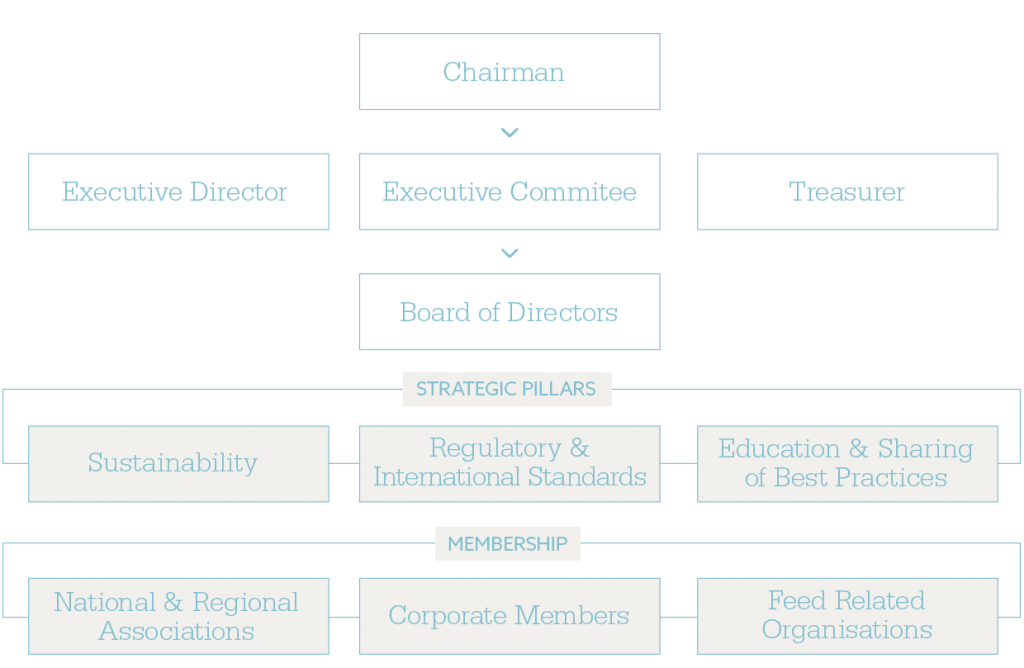
FIPAN’s Strategic Activities
FIPAN’s activities align with the NAFFP’s objectives to enhance feed production, safety, and sustainability. Key initiatives include:
1. Stakeholder Collaboration and Policy Development
FIPAN, as an IFIF member, played a pivotal role in formulating and validating the NAFFP, collaborating with academia, research institutes, and regulatory bodies like NIAS, NAFDAC, and SON. Its efforts ensure transparency and integrate international standards, supporting the policy’s goal of 20% annual feed production growth.
2. Representation and Industry Organization
FIPAN’s membership encompasses a comprehensive range of stakeholders within the feed industry, including feed manufacturers, raw material merchants, toll millers, equipment manufacturers and importers, minerals and vitamin premix producers, importers of feed concentrates and additives, raw material vendors, finished feed distributors, as well as affiliated institutions at both national and international levels.
3. Support for Feed Industry Operations
FIPAN promotes self-sufficiency through local feed milling equipment fabricators, supporting 650 small-scale mills. The NAFFP backs FIPAN’s efforts to scale up operations, addressing the 6.6–20 million metric ton market gap.
4. Advocacy for Feed Safety and Quality
FIPAN ensures compliance with NIAS, NAFDAC, SON, and Codex standards, addressing risks like aflatoxin transfer. This enhances traceability and consumer confidence, supporting domestic and export markets.
5. Market Expansion and Economic Impact
FIPAN supports SMEs, which account for over 50% of feed sector employment and value addition. Its focus on poultry and aquaculture drives economic growth, aligning with Nigeria’s livestock sector needs.
6. Capacity Building and Regulatory Compliance
FIPAN collaborates with the Federal Ministry of Livestock Development to train 1,000 stakeholders in nutrition, milling, and compliance, addressing raw material scarcity and high feed costs (70–75% of production expenses).
7. Environmental and Sustainability Focus
FIPAN promotes climate-smart practices, including local equipment to reduce emissions and alternative feed sources like crop residues and insects, across Nigeria’s six agro-ecological zones.
Strategic Path Moving Forward
FIPAN’s strategic vision, aligned with the NAFFP, focuses on fostering partnerships across the livestock value chain to unlock sustainable growth. By strengthening ties with government, regulatory bodies, and value chain partners, FIPAN aims to enhance feed availability, reduce costs, and support export market development
FIPAN will continue to advocate for infrastructure improvements, credit access, and innovation in feed production, ensuring the industry’s resilience against global and local shocks. The NAFFP’s target of 50 million metric tons by 2030 underscores FIPAN’s critical role in achieving food security and economic development.
Conclusion
The Feed Industry Practitioners Association of Nigeria (FIPAN) has emerged as a critical force in advancing Nigeria’s feed and livestock industry, playing a strategic role in implementing the National Animal Feed and Fodder Policy (NAFFP). Despite global and local economic headwinds—including inflation, supply chain disruptions, and infrastructural deficiencies—FIPAN has spearheaded initiatives that have driven growth in Nigeria’s feed production from 6.8 million metric tons in 2023 to 7.1 million metric tons in 2024, representing a modest yet resilient upward trajectory.
FIPAN’s alignment with international standards through its affiliation with the International Feed Industry Federation (IFIF), and its collaboration with regulatory agencies (NAFDAC, SON, NIAS), reflects its commitment to quality, safety, and sustainability. The organization has effectively unified a fragmented sector, integrating over 100 brands and supporting 650+ small-scale mills, which constitute 60% of national production capacity.
Furthermore, its focus on capacity building, SME support, climate-smart practices, and policy advocacy directly supports the NAFFP’s ambitious goal of 50 million metric tons of annual feed production by 2030. This positions Nigeria to significantly strengthen its livestock productivity, employment generation, and export potential, ultimately contributing to national food security and economic diversification.
FIPAN’s leadership, therefore, is not only facilitating the transformation of the feed sector but also anchoring Nigeria’s broader agricultural industrialization agenda, making it a cornerstone of sustainable development in the face of evolving global challenges.